Nitrogen Recovery in Wastewater Treatment
Look into how economic incentives, stringent limits, and emerging technologies are shaping nitrogen recovery in wastewater treatment.

newsletter
As environmental regulations tighten and climate targets intensify, wastewater treatment plants face mounting pressure to manage nitrogen more efficiently. Conventional removal methods in developed regions, though effective, are energy-intensive and could be carbon-heavy. By adopting recovery-oriented technologies, utilities can reduce their carbon footprint and operational costs while meeting nutrient discharge limits.
Nitrogen in Wastewater: Sources and Drivers for Recovery
Nitrogen is a building block of amino acids, nucleic acids, and chlorophyll, and is therefore indispensable for plant and animal growth. However, when released into the environment at high levels, nitrogen compounds, particularly ammonia and nitrate, cause water pollution, harm aquatic life, and contribute to greenhouse gas emissions.
Although agriculture remains the primary source of nitrogen entering water bodies, mainly through fertiliser use and livestock manure, wastewater discharges also contribute significantly. In regions with centralised wastewater treatment systems, nitrogen releases are generally better monitored and more effectively controlled.
Nitrogen Sources in a Wastewater Treatment Plant
In wastewater treatment facilities, nitrogen management primarily occurs in the biological (secondary) stage of the water line, with additional treatment needs arising during sludge handling, particularly after digestion and dewatering. The conventional processes that involve nitrogen include:
Nitrification – Ammonia (NH₃) is biologically oxidised to nitrite (NO₂⁻) and then to nitrate (NO₃⁻), by specialised bacteria under aerobic conditions. This process requires substantial aeration energy.
Denitrification – Nitrate is subsequently reduced nitrite then to nitrogen gas (N₂) by bacteria operating under anoxic (oxygen-free) conditions, using organic carbon as an electron donor. The resulting nitrogen gas is harmlessly released to the atmosphere.
Sidestream treatment – After sludge digestion and during dewatering, liquids separated from the biosolids, known as centrate, filtrate, liquors or reject water, contain high concentrations of ammonia. These recycle streams typically account for only 1–2% of the total plant flow but can contribute around 20–25% of the total ammonia load when returned to the headworks for treatment. Managing these streams effectively is therefore critical to maintaining stable nitrogen removal and overall plant performance.
A schematic showing the sources of nitrogen or nitrogen compounds in a typical wastewater treatment plant is shown below:
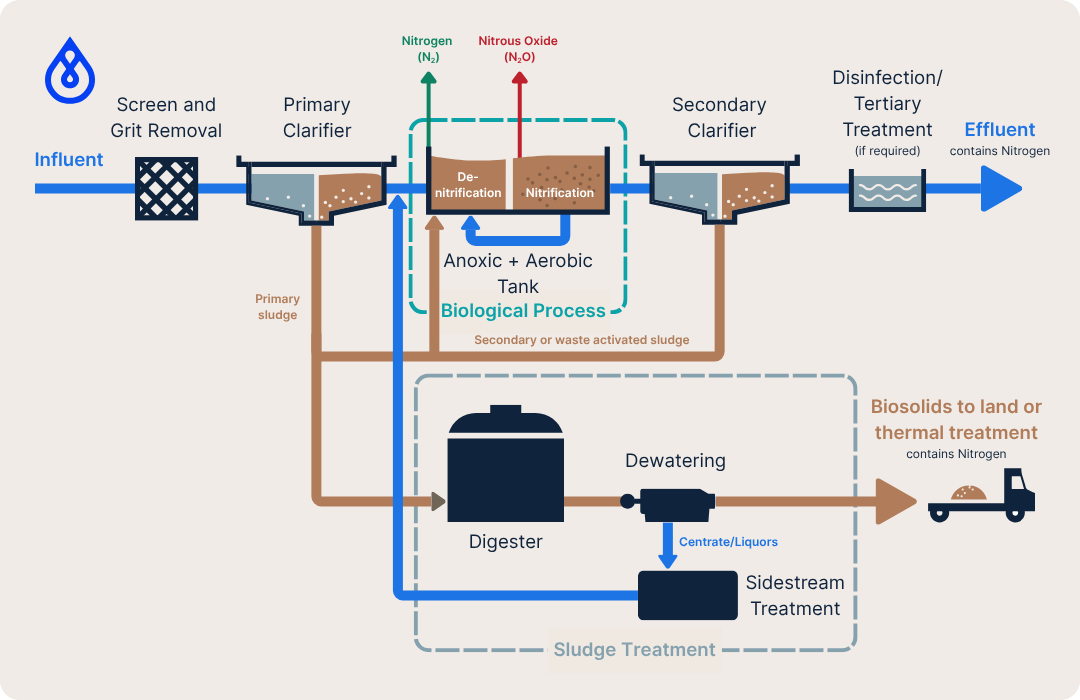
Aeration during nitrification is typically the single largest energy user in wastewater plants, often 45–75% of total plant electricity. While the combined nitrification and denitrification processes are effective at reducing nitrogen concentrations, they are also energy- and resource-intensive.
Growing Interest in Nitrogen Recovery
Across Europe and North America, increasingly stringent regulations have limited the amount of nitrogen that can be discharged into receiving waters. Meeting these low limits using conventional biological treatment often requires extended aeration, which substantially increases energy consumption; additional tank capacity, which many existing plants lack space for; and costly infrastructure upgrades.
As a result, wastewater utilities are seeking more cost-effective and sustainable solutions to meet nitrogen discharge permits without expanding secondary treatment capacity.
Beyond regulatory compliance, several other factors are driving the growing interest in nitrogen recovery:
- Climate impact of conventional removal: Biological nitrogen removal processes emit nitrous oxide (N₂O), a greenhouse gas with nearly 300 times the global warming potential of CO₂. Reducing reliance on these pathways can therefore help lower a plant’s carbon footprint.
- Resource value and fertiliser security: Nitrogen in wastewater exists in forms (such as ammonia) that can be captured and converted into valuable fertiliser products. Since synthetic fertiliser production relies on the energy-intensive Haber–Bosch process and global supply chains, recovering nitrogen from wastewater contributes to resource efficiency and nutrient circularity.
- Economic benefits for utilities: Sidestream treatment or recovery can reduce aeration demands in the main process, lower the need for chemical additives, and, when product quality standards are met, enable the production of saleable fertiliser products.
Nitrogen Recovery Technologies and Methods
Recovering nitrogen from wastewater is significantly more complex than simply removing it, but a growing suite of technologies now makes it possible to capture ammonia in useful forms.
In most wastewater treatment plants, recovery processes are applied to sidestream liquors—also known as centrate, filtrate, or reject water from sludge dewatering—because these contain much higher concentrations of ammonia than the main treatment stream. This makes recovery in sidestreams more energy- and cost-efficient.
Below are the principal approaches currently in practice or under development. Among these, deammonification is the most established, while other methods are in pilot stages. Many utilities are now exploring combinations of these technologies to improve overall performance and resource recovery.
Deammonification (Partial Nitritation-Anammox or PN/A)
While deammonification is primarily used for nitrogen removal, not recovery, ongoing research aims to integrate it with downstream recovery technologies and extend its application to mainstream municipal treatment.
It integrates partial nitritation (oxidising part of the ammonium to nitrite) with anaerobic ammonium oxidation (anammox), a process where specialised bacteria convert ammonium and nitrite directly into nitrogen gas.
This process represents an energy-efficient shortcut to conventional nitrification–denitrification, achieving the same end result without the need for organic carbon and with much lower oxygen requirements. Consequently, aeration energy use can be reduced by up to 60% compared to traditional biological nitrogen removal.
Chemical Precipitation
Chemical precipitation is primarily recognised as a phosphorus recovery technology, though it can also capture nitrogen—particularly in the form of ammonium—as a valuable co-benefit. The best-known example is struvite precipitation, which is the formation of magnesium ammonium phosphate (MAP).
Other precipitation-based recovery technologies are under development, including those that produce ammonium sulphate, a widely used nitrogen fertiliser that can be directly reused in agriculture.
The following methods and technologies include those that are either in early stages of development or those not yet commercially widespread in the wastewater industry:
Ammonia Stripping (Air Stripping)
This method removes ammonia by raising the pH to convert ammonium ions (NH₄⁺) into gaseous ammonia (NH₃), which is then stripped out using air or steam. The released ammonia is captured in an acid scrubber, typically using sulphuric acid, to produce ammonium sulphate, a valuable fertiliser.
The process is simple and well-established, offering reliable ammonia removal, but it is also energy-intensive and requires careful handling of caustic and acidic chemicals.
Of particular note is the development of a heat-driven ammonia stripping technology that uses thermal energy, rather than acids, to volatilise ammonia from wastewater.
While air stripping has long been used in industries such as power generation, it has only recently gained traction in the wastewater sector as a viable nitrogen recovery method.
Ion Exchange and Adsorption
In this process, ammonium is removed from wastewater using specialised media that selectively captures it. Once the media are saturated, they can be regenerated with a salt or alkaline solution, producing a smaller, concentrated ammonium stream that can be further processed into fertiliser products.
Because the media can be reused, these processes are efficient and flexible, making them particularly well suited for effluent polishing or sidestreams with low ammonia concentrations. However, a key challenge lies in managing the ammonium-rich regeneration by-product, which requires additional treatment before it can be converted into a usable form.
There are several other nitrogen recovery technologies currently at the pilot stage that are not discussed here in detail, such as membrane ammonia capture, electrochemical stripping, electrodialysis, capacitive deionisation, and algae cultivation.
In addition, fractional distillation, a method now under early deployment in the United States, is being explored to recover nitrogen from biosolids and manure, rather than from liquid sidestreams.
Nitrogen from Wastewater: Policy and Progress
Nitrogen recovery is advancing unevenly worldwide, increasingly steered by circular economy policy. Europe is currently setting the pace. The 2024 recast Urban Wastewater Treatment Directive requires tighter nutrient control through expanded tertiary treatment with stricter nitrogen and phosphorus removal, staged across the 2030s and extended to most large and medium plants by 2045. These are changes that reinforce the shift from treatment works to resource-recovery hubs.
EU product law now explicitly recognises certain recovered fertilisers: under the amended regulation for fertilisers, recovered high-purity materials, including ammonium salts, can be placed on the EU market as CE-marked fertilising products when purity and input-source criteria are met.
Despite strong drivers, deployment still faces economic, technical and institutional hurdles. Products such as ammonium sulfate compete in low-margin commodity markets; without sufficient ammonia concentration, favourable energy balances and credit for avoided treatment costs, revenues alone may not cover capital and operating costs.
Conversely, several studies indicate that, in the right conditions (e.g., high-strength sidestreams, integrated energy recovery, supportive tariffs or EPR), nitrogen recovery can achieve positive economics, while being environmentally beneficial. Integration must also protect effluent compliance and manage added process complexity.
As circular policies evolve, legal frameworks for recycled fertilisers develop, and technologies become more standardised, nitrogen recovery is likely to play an increasing role in sustainable wastewater management. By reducing eutrophication risks, supporting climate and energy objectives, and strengthening fertiliser resilience, it contributes to a more balanced and resource-efficient approach to nutrient management.
Nitrogen Recovery and the Thermal Hydrolysis Process
In plants that use the thermal hydrolysis process (THP), high temperature and pressure break down and solubilise organic solids. During subsequent anaerobic digestion (AD), the solubilised organic nitrogen is mineralised to ammonium, which typically raises ammonium levels in the dewatering centrate/reject liquor compared with conventional AD.
To manage this, some THP facilities apply sidestream liquor treatment. The most common approach is partial nitritation/anammox (PN/A), which can typically achieve around 80–85% total nitrogen removal in high-strength sidestreams.
As interest in targeted nitrogen recovery grows, several THP facilities are exploring emerging recovery-based processes such as ammonia stripping. Byosis, in collaboration with Cambi, is implementing such technology at the VEAS wastewater treatment plant in Oslo, with commissioning planned for late 2026.
As THP concentrates nitrogen in sludge liquors, which raises sidestream loads, that same concentration may enable efficient treatment and create strong opportunities for nitrogen recovery.
Curious how wastewater facilities are recovering phosphorus — another valuable nutrient — and the role THP plays in enabling it? Read our blog on Phosphorus Recovery in Wastewater Treatment.
Thermal Sludge Treatment: Methods and Advantages
Compare thermal sludge treatment methods - incineration, pyrolysis, and gasification - and learn about technologies improving their energy efficiency.
Dive inA 2026 Guide to Key Water, Wastewater, Biosolids Conferences
Dive into our list of the most anticipated water, wastewater, and biosolids conferences of the year.
Dive inPFAS in Biosolids: Solutions and Treatment Technologies
See how wastewater and sludge treatment plants are managing PFAS in biosolids through regulation and advanced technologies.
Dive in